

From First-in-Human Studies to Post-Market Surveillance
Medical device trials require a different level of adaptability, and oversight compared to traditional drug trials. oomnia offers an all-in-one system designed to streamline operations and to ensure compliance with device-specific regulations. With integrated modules like EDC, CTMS, eTMF, ePRO, eConsent and real-time monitoring, oomnia supports your entire trial lifecycle and simplifies complex workflows.

Every device trial is unique. oomnia accompanies you through all market and trial phases.
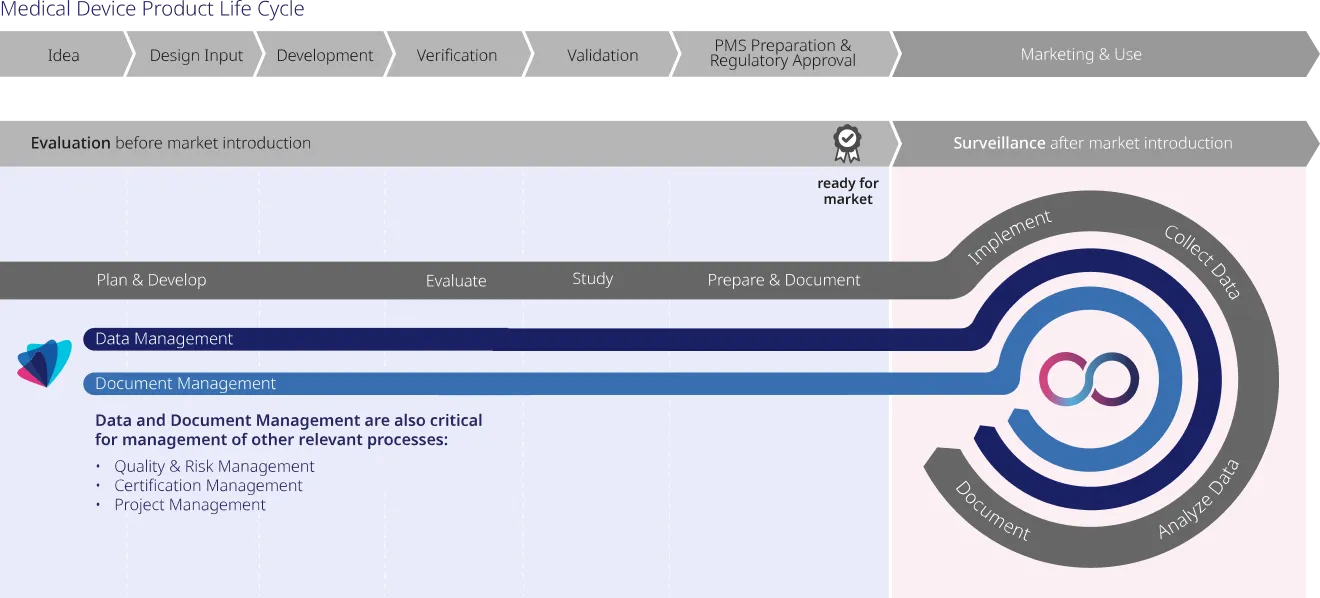

Prior to market entry, medical device manufacturers undergo essential clinical evaluations to verify product safety, efficacy, and market readiness. This process includes clinical investigations, scientific literature reviews, and preclinical data assessments. Using our software, you are fully prepared for ISO 13485 certification, ensuring compliance with all the requirements of this standard. Additionally, our platform guarantees adherence to the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), providing comprehensive support for your regulatory needs.

After entering the market, medical device manufacturers must actively monitor their products‘ safety and performance through post-market surveillance. This phase demands systematic data collection, analysis, and interpretation to identify necessary corrective actions and ensure ongoing compliance with regulatory standards. oomnia excels in managing this continuous process, offering comprehensive data management tools for effective surveillance and adherence to regulations.

Eager to Explore Tailored Services?
Discover how our clinical trial services can support your trial research. We combine hands-on expertise with smart tools to help you run faster, smoother, and more compliant trials.
Tell Us About Your TrialBenefit from a unified approach, real-time insights, and the expertise of our team for your Medical Device, Software as a Medical Device (SaMD), and PMCF studies.
Enjoy the ease of automated compliant datasets, audit trails, and detailed reporting, all ensuring effortless adherence to regulations throughout your medical device trial.
oomnia seemlessly integrates and handles various data types, including imaging, biometric data, and data extracted from Electronic Health Records (EHRs).
oomnia enables efficient post-market surveillance with swift capturing and analysis of real-world evidence. This ensures continuous monitoring of your device’s safety and performance.
oomnia enhances interoperability and enables real-time data sharing and seamless collaboration across systems and teams.
As ISO 27001 certified experts, we built oomnia with top-tier security, strict access controls, and default HIPAA and GDPR compliance.
oomnia scales with your needs. From small pilot studies to complex international trials and PMCF, all backed by expert services at every step.
oomnia offers smart statistical analysis and reporting tools to support regulatory submissions with built-in visualizations and export-ready outputs.
Get high data integrity through built-in validation and cleaning tools. It delivers accurate and reliable data for decision-making and regulatory readiness.

Switch to oomnia Now
Tell us about your project and let’s explore how we can support it.
Start Your Journey With Us
oomnia Adapts to Every Study Type
oomnia is built to handle a wide range of trial designs and therapeutic areas. Whether you are running traditional drug trials, decentralized studies, combination product research, or registry-based investigations, oomnia adapts to your specific workflows, compliance needs, and scale. Explore how our technology helps streamline operations, reduce manual work, and ensure data consistency across all types of clinical studies.





You decide where to store your data and who and how to access it. We fulfill all regulatory standards for secure data storage and exchange.
Explore the next level of comprehensive knowledge database and real-time metrics to generate new insights and assets.
All clinical trial tools are integrative parts of one cohesive system. You learn, collaborate, and report in real time.
